Human IL-17 (Interleukin 17) CLIA Kit
| Size: | |
| Qty: |
-
+
|
| Price: | $682 |
Reactivity: Human
Detection Range: 12.50~800 pg/mL
Sensitivity: 7.50 pg/mL
Add to cartIntended use
This CLIA kit applies to the in vitro quantitative determination of Human IL-17 concentrations in serum, plasma and other biological fluids.
Test principle
This CLIA kit uses the Sandwich-CLIA principle. The micro CLIA plate provided in this kit has been pre-coated with an antibody specific to Human IL-17. Standards or samples are added to the micro CLIA plate wells and combined with the specific antibody. Then a biotinylated detection antibody specific for Human IL-17 and Avidin-Horseradish Peroxidase (HRP) conjugate are added successively to each micro plate well and incubated. Free components are washed away. The substrate solution is added to each well. Only those wells that contain Human IL-17, biotinylated detection antibody and Avidin-HRP conjugate will appear fluorescence. The Relative light unit (RLU) value is measured by the Chemiluminescence immunoassay analyzer. The RLU value is positively associated with the concentration of Human IL-17. You can calculate the concentration of Human IL-17 in the samples by comparing the RLU value of the samples to the standard curve.
| Assay type | Sandwich-CLIA |
| Format | 96T |
| Assay time | 3.5h |
| Reactivity | Human |
| Detection method | Chemiluminescence |
| Detection range | 12.50-800 pg/mL |
| Sensitivity | 7.50 pg/mL |
| Sample volume | 100μL |
| Sample type | serum, plasma and other biological fluids |
| Repeatability | CV < 15% |
Specificity
This kit recognizes Human IL-17 in samples. No significant cross-reactivity or interference between Human IL-17 and analogues was observed.
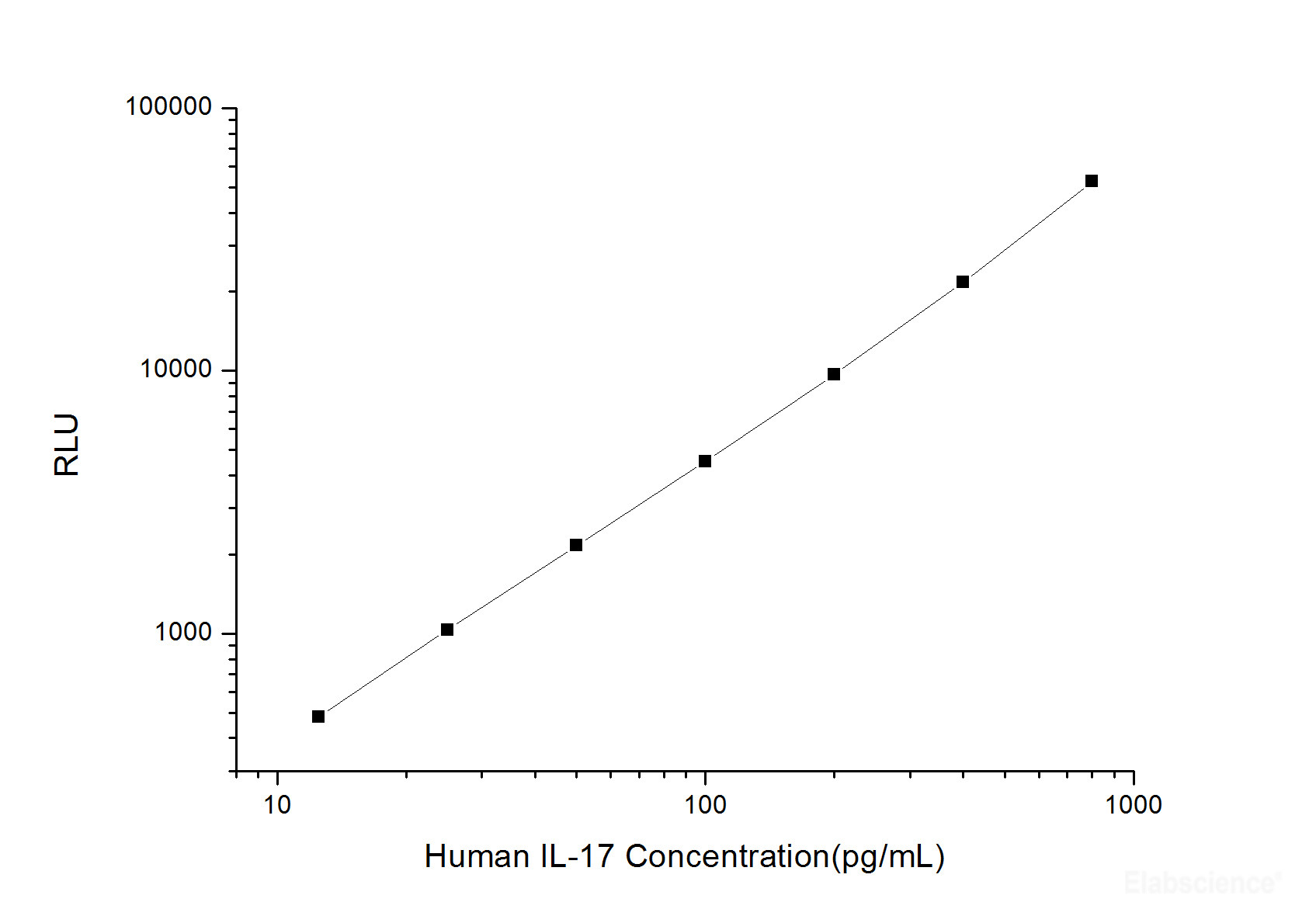
Typical data
As the RLU values of the standard curve may vary according to the conditions of the actual assay performance (e.g. operator, pipetting technique, washing technique or temperature effects), the operator should establish a standard curve for each test. Typical standard curve and data is provided below for reference only.
| Concentration (pg/mL) |
RLU | Average | Corrected |
|---|---|---|---|
| 800 | 49510 55774 |
52642 | 52616 |
| 400 | 21468 22000 |
21734 | 21708 |
| 200 | 10538 8878 |
9708 | 9682 |
| 100 | 4264 4840 |
4552 | 4526 |
| 50 | 2295 2081 |
2188 | 2162 |
| 25 | 1139 981 |
1060 | 1034 |
| 12.50 | 489 529 |
509 | 483 |
| 0 | 26 26 |
26 | -- |
|
Precision
Intra-assay Precision (Precision within an assay): 3 samples with low, mid range and high level Human IL-17 were tested 20 times on one plate, respectively.
Inter-assay Precision (Precision between assays): 3 samples with low, mid range and high level Human IL-17 were tested on 3 different plates, 20 replicates in each plate.
| Intra-assay Precision | Inter-assay Precision | |||||
|---|---|---|---|---|---|---|
| Sample | 1 | 2 | 3 | 1 | 2 | 3 |
| n | 20 | 20 | 20 | 20 | 20 | 20 |
| Mean (pg/mL) | 42.68 | 66.99 | 285.27 | 44.38 | 65.34 | 282.26 |
| Standard deviation | 4.08 | 7.60 | 31.89 | 4.29 | 6.61 | 26.76 |
| C V (%) | 9.56 | 11.34 | 11.18 | 9.67 | 10.12 | 9.48 |
Recovery
The recovery of Human IL-17 spiked at three different levels in samples throughout the range of the assay was evaluated in various matrices.
| Sample Type | Range (%) | Average Recovery (%) |
|---|---|---|
| Serum (n=5) | 97-114 | 104 |
| EDTA plasma (n=5) | 99-114 | 105 |
| Cell culture media (n=5) | 89-102 | 95 |
Linearity
Samples were spiked with high concentrations of Human IL-17 and diluted with Reference Standard & Sample Diluent to produce samples with values within the range of the assay.
| Serum (n=5) | EDTA plasma (n=5) | Cell culture media (n=5) | ||
|---|---|---|---|---|
| 1:2 | Range (%) | 88-103 | 101-118 | 91-107 |
| Average (%) | 95 | 108 | 98 | |
| 1:4 | Range (%) | 87-103 | 91-108 | 95-108 |
| Average (%) | 94 | 99 | 101 | |
| 1:8 | Range (%) | 88-103 | 86-96 | 86-99 |
| Average (%) | 96 | 91 | 91 | |
| 1:16 | Range (%) | 88-103 | 93-108 | 101-117 |
| Average (%) | 95 | 99 | 108 |
Kit Components& Storage
An unopened kit can be stored at 4℃ for 1 month. If the kit is not used within 1 month, store the items separately according to the following conditions once the kit is received.
| Item | Specifications | Storage |
|---|---|---|
| Micro CLIA Plate(Dismountable) | 8 wells ×12 strips | -20℃, 6 months |
| Reference Standard | 2 vials | |
| Concentrated Biotinylated Detection Ab (100×) | 1 vial, 120 μL | |
| Concentrated HRP Conjugate (100×) | 1 vial, 120 μL | -20℃(shading light), 6 months |
| Reference Standard & Sample Diluent | 1 vial, 20 mL | 4℃, 6 months |
| Biotinylated Detection Ab Diluent | 1 vial, 14 mL | |
| HRP Conjugate Diluent | 1 vial, 14 mL | |
| Concentrated Wash Buffer (25×) | 1 vial, 30 mL | |
| Substrate Reagent A | 1 vial, 5 mL | 4℃ (shading light) |
| Substrate Reagent B | 1 vial, 5 mL | 4℃ (shading light) |
| Plate Sealer | 5 pieces | |
| Product Description | 1 copy | |
| Certificate of Analysis | 1 copy |
Note: All reagent bottle caps must be tightened to prevent evaporation and microbial pollution.
The volume of reagents in partial shipments is a little more than the volume marked on the label, please use accurate measuring equipment instead of directly pouring into the vial(s).
Other supplies required
- Chemiluminescence immunoassay analyzer
- High-precision transfer pipette, EP tubes and disposable pipette tips
- Incubator capable of maintaining 37℃
- Deionized or distilled water
- Absorbent paper
- Loading slot for Wash Buffer
Assay procedure
|
1.Add 100 μL of standard or sample to each well. Incubate for 90 min at 37℃. |
|

2.Remove the liquid. |
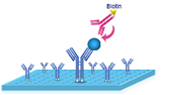
|

3.Add 100 μL Biotinylated Detection Ab. Incubate 1 hour at 37℃. Aspirate and wash 3 times. |
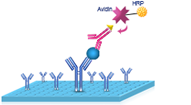
|

4.Add 100 μL HRP Conjugate. Incubate 30 min at 37℃. Aspirate and wash 5 times. |

|

5.Add 100 μL Substrate Mixture Solution. Incubate for 5 min at 37℃. |
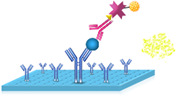
|

6.Fluorescence appeared. Measure the RLU value with the Chemiluminescence immunoassay analyzer. Calculation of the results. |